Efficacy Data
PRIMARY ENDPOINT
REXULTI® (brexpiprazole): Proven to reduce the FREQUENCY of agitation symptoms
Study 6 and 7: REXULTI 2 or 3 mg/day arm was statistically significantly superior to placebo in mean change from baseline in the CMAI total score at Week 12
Study 7 results
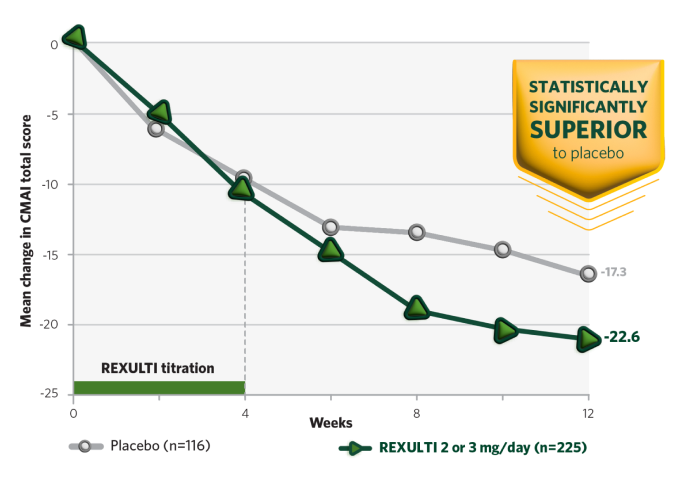
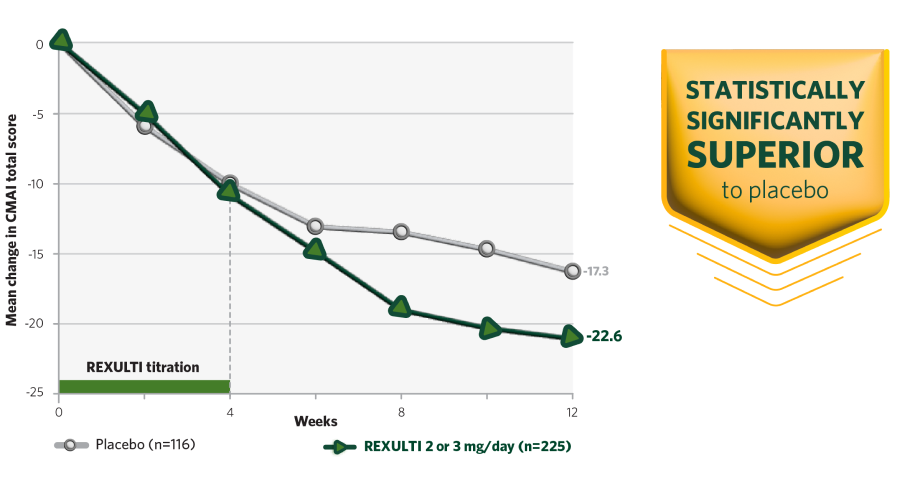
CMAI, Cohen-Mansfield Agitation Inventory.
STUDY 6 AND 7 DESIGN AND EFFICACY SUMMARY
REXULTI was studied in 2 Phase III, 12-week, randomized, double-blind, placebo-controlled, fixed-dose pivotal trials evaluating frequency of agitation symptoms and safety profile in patients with dementia due to Alzheimer’s disease. After a screening phase of 6 weeks, patients titrated for 2 to 4 weeks to their assigned dose.1,2
Primary endpoint was change in agitation symptom frequency (CMAI total score) from baseline at Week 12 in both studies.
REXULTI pivotal studies: Two Phase III, 12-week, randomized, double-blind, placebo-controlled fixed-dose studies evaluated frequency (CMAI total score) of agitation symptoms in patients with dementia due to Alzheimer's disease1,2
Study 6: Evaluated REXULTI 1 mg/day (n=134) or 2 mg/day (n=138), or placebo (n=131). Titration began at 0.25 mg/day for Days 1−3, then increased to 0.5 mg/day at Days 4−14, 1 mg/day at Days 15−28, and maintained at either 1 or 2 mg/day from Day 29 onward depending on assigned dose.1
Study 7: Evaluated REXULTI 2 mg/day or 3 mg/day (n=228), or placebo (n=117). Titration began at 0.5 mg/day for Days 1−7, then increased to 1 mg/day at Days 8−14, 2 mg/day at Days 15−28, and either maintained at 2 mg/day or increased to 3 mg/day from Day 29 onward.2
Key inclusion criteria1,2
- Probable Alzheimer's disease diagnosis as per NINCDS-ADRDA Criteria
- Agitation as determined by NPI NH A/A score ≥4
- MMSE: ≥5 and ≤22
- Exhibit sufficient agitation behaviors at time of entry to warrant use of pharmacotherapy, after excluding other factors
Additional inclusion criteria in Study 7
- Met criteria for agitation as defined by the IPA provisional definition
- Aggressive agitation at baseline (≥1 CMAI Factor 1 behavior)
Concomitant medications
- Cholinesterase inhibitors, memantine, and other cognitive enhancers, as well as antidepressants (like SSRI or SNRI), were permitted for the duration of the studies; doses had to be stable prior to and during the study

PRIMARY ENDPOINT
Primary endpoint was change in agitation symptom frequency (CMAI total score) from baseline at Week 12 in both studies.
Baseline characteristics1,2
Baseline demographic and clinical characteristics were similar across the REXULTI and placebo groups within Studies 6 and 7.1,2
CMAI, Cohen-Mansfield Agitation Inventory; IPA, International Psychogeriatric Association; MMSE, Mini-Mental State Examination; NINCDS-ADRDA, National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association; NPI-NH A/A, Neuropsychiatric Inventory − Nursing Home version, Agitation/aggression domain; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Study 6 and 7 Results1,2:
aDosages statistically significantly superior to placebo.
SECONDARY ENDPOINT
REXULTI: Change in frequency across subscales of agitation symptoms
REXULTI was approved for the treatment of agitation associated with dementia due to Alzheimer’s disease based on the primary endpoint, change in CMAI total score from baseline at Week 12.
A secondary endpoint was a change from baseline at Week 12 in CMAI subscale scores.
bIn a supplementary analysis to examine the magnitude and direction of CMAI subscale response, Factor 1 (aggressive behavior), Factor 2 (physically non-aggressive behavior), and Factor 3 (verbal agitation) scores trended in the same direction with no single factor overly influencing the CMAI total score.
Agitated behaviors as defined by CMAI4
The Cohen-Mansfield Agitation Inventory (CMAI) is a clinically validated scale measuring the frequency of 29 agitated behaviors.
- Grouped into 3 subscales
- Scored by clinicians based on caregiver input
VERBALLY AGITATED
- Complaining
- Constant unwarranted request
for attention or help
- Repetitive sentences or questions
- Negativism
PHYSICALLY NON-AGGRESSIVE
- Pacing, aimless wandering
- General restlessness
- Inappropriate dress or disrobing
- Trying to get to a different place
- Handling things inappropriately
- Performing repetitive mannerisms
AGGRESSIVE
- Screaming
- Biting
- Hitting
- Kicking
- Hurting self or others
- Cursing or verbal aggression
- Pushing
- Scratching
- Throwing things
- Spitting
- Tearing things/destroying property
- Grabbing onto people

Additional behaviors assessed by CMAI total score that often have low rates of occurrence include making physical sexual advances, intentional falling, eating/drinking inappropriate substances, hiding things, hoarding things, making verbal sexual advances, and strange noises (weird laughter or crying).4,5
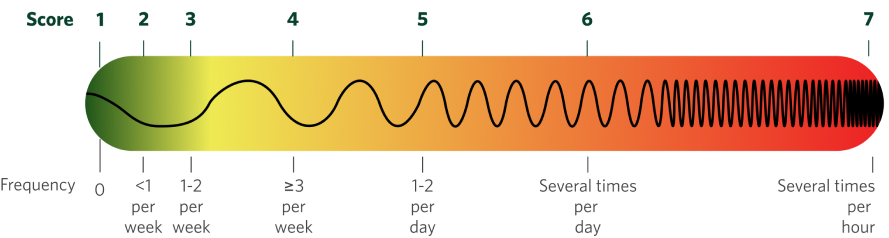
What is a CMAI total score?4
- Each of these 29 agitated behaviors is assigned a frequency score based on its frequency through the preceding 2 weeks
- The sum of each agitated behavior's frequency score creates a CMAI total score
- A negative change in total CMAI score indicates improvement
Swipe to see full chart
References: 1. Grossberg GT, Kohegyi E, Mergel V, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer's dementia: two 12-week, randomized, double-blind, placebo-controlled trials. Am J Geriatr Psychiatry. 2020;28(4):383-400. 2. Lee D, Slomkowski M, Hefting N, et al. Brexpiprazole for the treatment of agitation in Alzheimer dementia: a randomized clinical trial. JAMA Neurol. 2023;80(12):1307-1316. 3. Data on file (REX-283). 4. Cohen-Mansfield J. Agitated behavior in persons with dementia: the relationship between type of behavior, its frequency, and its disruptiveness. J Psychiatr Res. 2008;43(1):64-69. 5. Rabinowitz J, Davidson M, De Deyn PP, et al. Factor analysis of the Cohen-Mansfield Agitation Inventory in three large samples of nursing home patients with dementia and behavioral disturbance. Am J Geriatr Psychiatry. 2005;13(11):991-998.
