When added to an antidepressant,
REXULTI® (brexpiprazole) 2 mg/day was superior in reducing mean MÅDRS total score vs antidepressant + placebo
Mean Change from Baseline in MÅDRS Total Score by Study Visit (Week) in
Patients with MDD in Adults (Study 1)1
Mean Change from Baseline in MÅDRS Total Score by Study Visit (Week) in Patients with MDD in Adults (Study 1)1
It is unknown if the differences observed at time points earlier than Week 6 represent clinically relevant treatment effects.
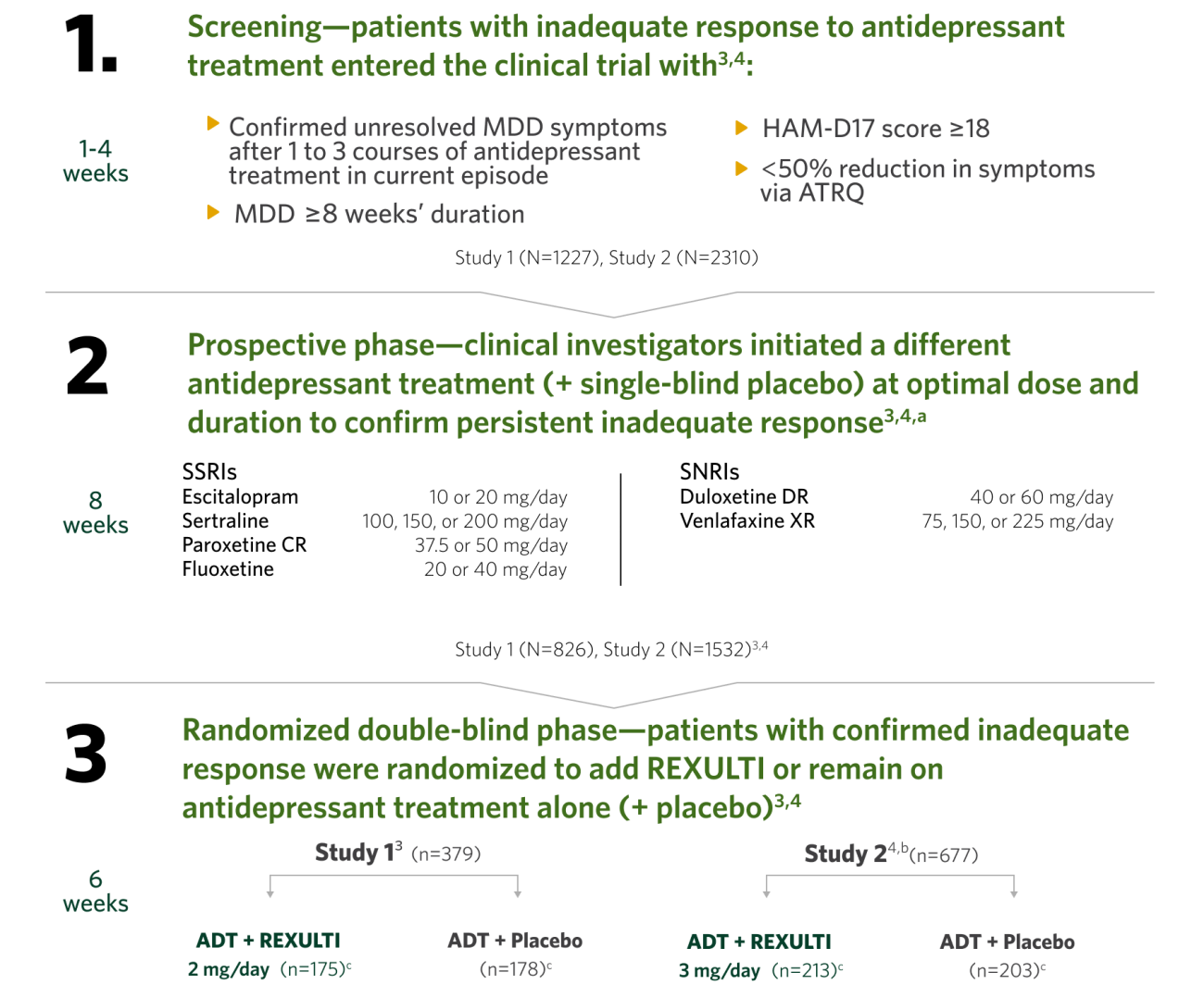
aNot all patients had an inadequate response to antidepressant alone in the 8-week prospective phase. Only patients with an inadequate response entered the randomized double-blind treatment phase.
*p<0.001
Mean MÅDRS before prospective treatment (SD): 31.0 (4.7). Mean MÅDRS after prospective treatment (SD): 27.1 (5.7). Mean MÅDRS at randomization (SD): ADT + placebo (n=178), 27.3 (5.6); ADT + REXULTI 2 mg/day (n=175), 26.9 (5.7).1
MDD study design and efficacy summary
The efficacy of REXULTI in the adjunctive treatment of major depressive disorder (MDD) was evaluated in two 6-week, double-blind, placebo-controlled, fixed-dose studies of adult patients meeting DSM-IV-TR criteria for MDD, with or without symptoms of anxiety, who had an inadequate response to prior antidepressant therapy (1 to 3 courses) in the current episode. After a screening phase of 1-4 weeks, patients entered into an 8-week prospective treatment phase with an SSRI or SNRI (+ single-blind placebo). Subsequently, patients having persistent symptoms without substantial improvement throughout the course of treatment and who met inclusion criteria were randomized to receive adjunctive REXULTI or placebo.3,4
In Study 2 for the REXULTI (3 mg/day) + ADT treatment group, the placebo-subtracted difference was -2.0 with a 95% CI (-3.4, -0.5).
Primary endpoint was the mean change from baseline to Week 6 in the MÅDRS total score in the randomization phase.3,4
In the second pivotal trial, at the 3 mg/day maximum dose, the mean change from baseline (SE) at 6 weeks (randomized phase) was -8.3 (0.5) for ADT + REXULTI (n=213) vs -6.3 (0.5) for ADT + placebo (n=203), and the MÅDRS baseline (SD) for ADT + REXULTI and ADT + placebo was 26.5 (5.3) and 26.5 (5.2), respectively.4
The efficacy and safety of REXULTI were also studied in patients randomized to receive 1 mg/day in Study 2 (n=211). Results for the ADT + REXULTI 1 mg group for the primary efficacy parameter were not statistically significant when compared with ADT + placebo.4
ADT, antidepressant treatment; ATRQ, Antidepressant Treatment Response Questionnaire; CR, controlled release; DR, delayed release; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Text Revision); HAM-D17, 17-Item Hamilton Depression Rating Scale; MÅDRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; SD, standard deviation; SE, standard error; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; XR, extended release.
Contraindication
In patients with known hypersensitivity to brexpiprazole or any of its components. Reactions have included: rash, facial swelling, urticaria, and anaphylaxis.