Dosing information for REXULTI® (brexpiprazole) in adults and pediatric patients 13+ with schizophrenia
Dosing and titration schedule
REXULTI—recommended target dose: 2 mg/day to 4 mg/day
Adults:
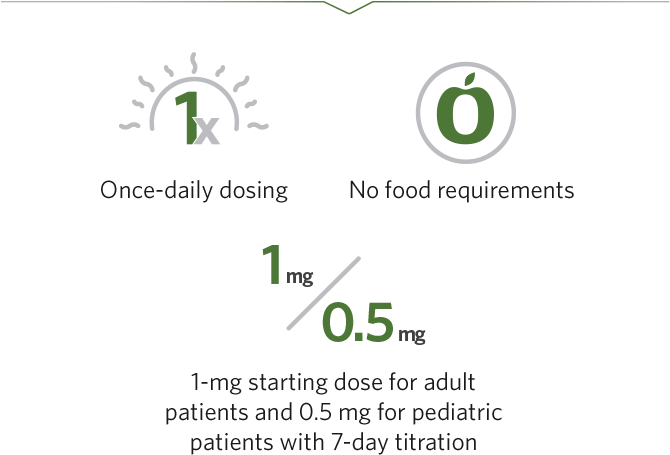
- Recommended starting dosage for REXULTI: 1 mg once daily on Days 1 to 4, taken orally with or without food
- Titrate to 2 mg once daily on Day 5 through Day 7, then to 4 mg on Day 8 based on the patient’s clinical response and tolerability
- Recommended target dosage: 2 mg to 4 mg once daily
- Maximum recommended daily dosage: 4 mg
Pediatric patients (13 to 17 years of age):
- Recommended starting dosage for REXULTI: 0.5 mg once daily on Days 1 to 4, taken orally with or without food
- Titrate to 1 mg once daily on Day 5 through Day 7, then to 2 mg on Day 8 based on the patient’s clinical response and tolerability
- Weekly dose increases can be made in 1-mg increments
- Recommended target dosage: 2 mg to 4 mg once daily
- Maximum recommended daily dosage: 4 mg
Adults:
- Recommended starting dosage for REXULTI: 1 mg once daily on Days 1 to 4, taken orally with or without food
- Titrate to 2 mg once daily on Day 5 through Day 7, then to 4 mg on Day 8 based on the patient’s clinical response and tolerability
- Recommended target dosage: 2 mg to 4 mg once daily
- Maximum recommended daily dosage: 4 mg
Pediatric patients (13 to 17 years of age):
- Recommended starting dosage for REXULTI: 0.5 mg once daily on Days 1 to 4, taken orally with or without food
- Titrate to 1 mg once daily on Day 5 through Day 7, then to 2 mg on Day 8 based on the patient’s clinical response and tolerability
- Weekly dose increases can be made in 1-mg increments
- Recommended target dosage: 2 mg to 4 mg once daily
- Maximum recommended daily dosage: 4 mg
Dose adjustments for REXULTI
- Dose adjustments may be needed in patients with hepatic or renal impairment
- Administer half the dose when given with strong CYP2D6 or strong CYP3A4 inhibitors, or in patients who are known CYP2D6 poor metabolizers
- Administer a quarter of the dose with the concurrent use of both strong/moderate CYP2D6 inhibitors and strong/moderate CYP3A4 inhibitors. Likewise, administer a quarter of the dose in patients who are known CYP2D6 poor metabolizers taking strong/moderate CYP3A4 inhibitors
- Double the dose over 1 to 2 weeks when administering with strong CYP3A4 inducers
Download information on the titration options for initiating treatment with REXULTI in adults and pediatric patients ages 13 years and older with schizophrenia.
Consider starting your patients today


Sample starter packs now availablea
For your appropriate patients with schizophrenia, consider REXULTI sample titration packs:
- Single-dose packs:
0.5 mg, 1 mg, 2 mg, 3 mg, 4 mg
aSamples are not for sale or reimbursement.
