Demonstrated efficacy across both short-term and maintenance studies with REXULTI® (brexpiprazole)
Short-term efficacy data: 20-point reduction in PANSS total score from baseline with REXULTI 4 mg across 2 trials in adults
Post-hoc analysis of Study 3 and Study 4:
REXULTI 4 mg/day—reduction in PANSS total score vs placebo in pooled data at 6 weeks1
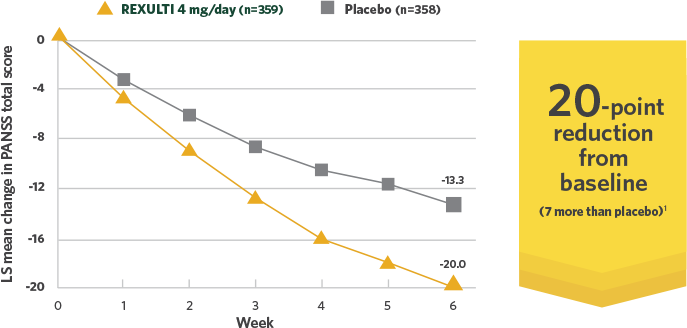
Post-hoc analysis of Study 3 and Study 4:
REXULTI 4 mg/day—reduction in PANSS
total score vs placebo in pooled data at 6 weeks1

Adapted with permission.
Pivotal Trial Design2
- Two 6-week, randomized, placebo-controlled, fixed-dose pivotal trials conducted in patients who met DSM-IV-TR criteria for SZ and would benefit from hospitalization or continued hospitalization for an acute exacerbation of symptoms2
- Treatment initiated at 1 mg/day on Days 1-4, titrated to 2 mg/day on Days 5-7, then maintained or increased to 4 mg/day on Day 8, depending on treatment arm
Primary endpoint was change in PANSS total score from baseline to Week 6.
Difference in mean reduction in PANSS total score vs placebo at Week 6 in both pivotal trials
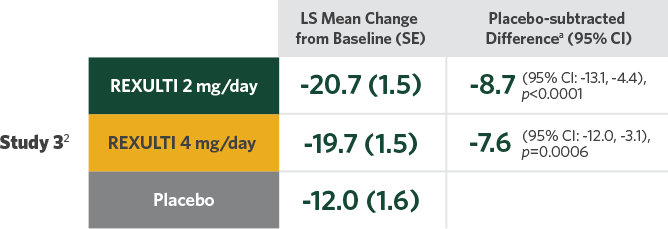
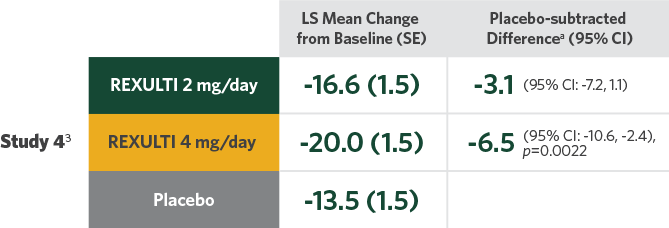
- Statistical significance was achieved at 2 mg/day vs placebo in Study 32
- A 1-mg/day treatment arm was included in Study 4. Statistical significance was not achieved for the primary endpoint with either the 1-mg/day dose or the 2-mg/day dose compared with placebo3
aDifference (drug minus placebo) in least-squares mean change from baseline.
CI, confidence interval; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Text Revision); LS, least squares; PANSS, Positive and Negative Syndrome Scale; SE, standard error; SZ, schizophrenia.
Contraindication
In patients with known hypersensitivity to brexpiprazole or any of its components. Reactions have included: rash, facial swelling, urticaria and anaphylaxis.
REXULTI 4 mg significantly decreased PANSS total score for patients who were markedly to moderately ill vs placebo across 2 trials in adults4,5
Study 3 and Study 4:
REXULTI—mean baseline PANSS total score at randomization and
mean change from baseline at 6 weeks4,5,a
The PANSS is a 30-item scale that measures positive symptoms of SZ (7 items), negative symptoms of SZ (7 items), and general psychopathology (16 items), with each rated on a scale of 1 (absent) to 7 (extreme); the total PANSS scores range from 30 (best) to 210 (worst).4
Study 3 and Study 4:
REXULTI—mean baseline PANSS total score at randomization and
mean change from baseline at 6 weeks4,5,a
The PANSS is a 30-item scale that measures positive symptoms of SZ (7 items), negative symptoms of SZ (7 items), and general psychopathology (16 items), with each rated on a scale of 1 (absent) to 7 (extreme); the total PANSS scores range from 30 (best) to 210 (worst).4
PANSS clinician-rated symptoms4:
Positive:
- Excitement
- Grandiosity
- Delusions
- Hostility
- Suspiciousness
- Hallucinatory behavior
- Conceptual disorganization
Negative:
- Blunted affect
- Difficulty in abstract thinking
- Lack of spontaneity and flow of conversation
- Poor rapport
- Stereotyped thinking
- Emotional withdrawal
- Passive-apathetic social withdrawal
General psychopathology:
- Anxiety
- Tension
- Uncooperativeness
- Poor impulse control
- Somatic concern
- Guilt feelings
- Mannerisms and posturing
- Depression
- Motor retardation
- Unusual thought content
- Disorientation
- Poor attention
- Lack of judgment and insight
- Disturbance of volition
- Preoccupation
- Active social avoidance
aPatients were hospitalized for screening and throughout the 6-week treatment phase.3
bMean baseline PANSS total scores (SD) for patients were Study 3: REXULTI 2 mg/day, 95.9 (13.8); REXULTI 4 mg/day, 94.7 (12.1); placebo, 95.7 (11.5); Study 4: REXULTI 2 mg/day, 96.3 (12.9); REXULTI 4 mg/day, 95.0 (12.4); placebo, 94.6 (12.8).3
cn=718; sum of patients in the efficacy analyses who received REXULTI 2 mg/day (n=359) or 4 mg/day (n=359) in Study 3 and Study 4.
dn=358; sum of patients in the efficacy analyses who received placebo in Study 3 and Study 4.
SD, standard deviation.
Study 5:
Demonstrated efficacy as maintenance treatment in adults
PANSS total score during stabilization and maintenance phases6
PANSS total score during stabilization and maintenance phases6
Mean baseline PANSS total scores [SD] (at Week 0 in the maintenance phase): REXULTI (n=97), 56.5 [8.7]; placebo (n=105), 58.1 [8.1].6
Patients had a PANSS total score of >80 and, if needed, were part of a 1- to 4-week washout phase of previous medications before moving on to stabilization.6
Study 5 trial design7
Single-blind stabilizationa
Patients with SZ were stabilized on flexible doses of REXULTI 1 mg/day to 4 mg/day for at least 12 consecutive weeks
Double-blind maintenance
Patients who met stabilizationa criteria and remained on a stable dose of REXULTI for at least the last 4 weeks were then randomized 1:1 for 52 weeks to either:
- Their achieved stable dose of REXULTI
or - Placebo
aStabilization was defined as all of the following: (1) outpatient status; (2) PANSS total score ≤70; (3) a PANSS score of ≤4 on conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content; (4) CGI-severity ≤4; (5) no current suicidal behavior; (6) no violent or aggressive behavior resulting in injury or property damage.6
CGI, Clinical Global Impression.
Important Warning and Precaution for Cerebrovascular Adverse Events, Including Stroke
In clinical trials, elderly patients with dementia randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. REXULTI is not approved for the treatment of patients with dementia-related psychosis without agitation associated with dementia due to Alzheimer’s disease.
Study 5:
REXULTI achieved a 71% reduced risk of impending relapse vs placebo
- Mean dose during maintenance phase was 3.6 mg/day6
- Primary endpoint—time from randomization to impending relapse in the maintenance phase
- Impending relapse was defined as any of the following:
- CGI-Improvement score of ≥5 and an increase to >4 on PANSS conceptual disorganization, hallucinatory behavior, suspiciousness, or unusual thought content, with either a ≥2 increase on a specific item or ≥4 increase on the combined 4 items
- Hospitalization due to worsening of psychotic symptoms
- Current suicidal behavior
- Violent/aggressive behavior
- Impending relapse was defined as any of the following:
Study 5: Reduced risk of impending relapse
aThe proportion of patients in the maintenance phase who experienced impending relapse with brexpiprazole was 13.5% compared with 38.5% for placebo.6
- The risk of impending relapse was calculated using a Kaplan-Meier estimation over the 52 weeks. The calculated hazard ratio (HR=0.292 [95% CI: 0.156, 0.548], p<0.0001) was used to estimate the reduction in risk of impending relapse for patients continuing treatment with REXULTI vs those given placebo6
- Trial was terminated early because maintenance of efficacy had been demonstrated
Of the 202 patients randomized, 2 patients were excluded from the efficacy analysis: 1 patient taking REXULTI did not have post-randomization efficacy evaluations, and 1 patient taking placebo did not take the investigational medicinal product.
HR, hazard ratio.
Important Warning and Precaution for Neuroleptic Malignant Syndrome (NMS)
NMS is a potentially fatal symptom complex reported in association with administration of antipsychotic drugs, including REXULTI. Clinical signs of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. Manage NMS with immediate discontinuation of REXULTI, intensive symptomatic treatment, and monitoring.
